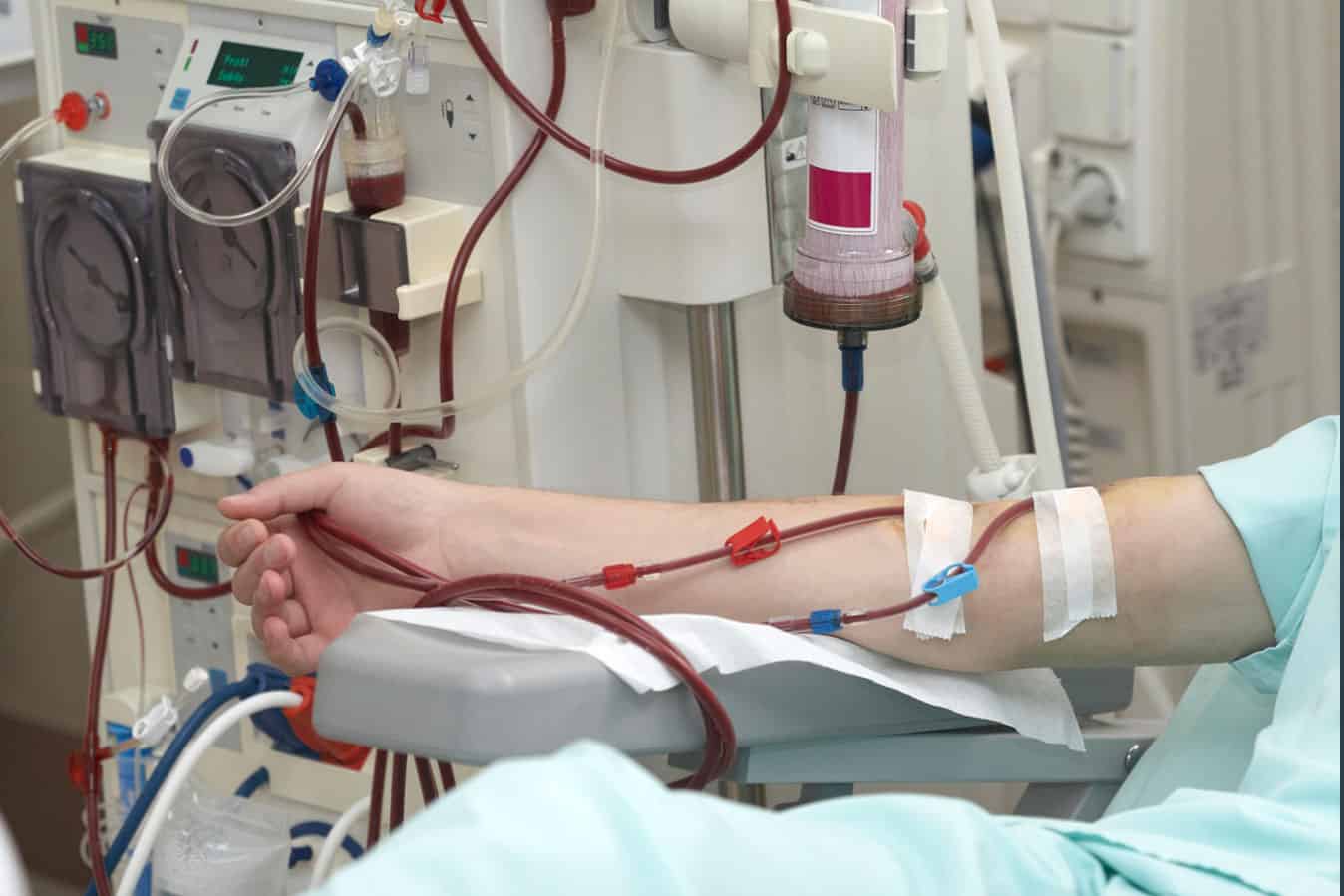
A simple change in practice for deceased donor kidney transplants could reduce the need for dialysis in transplant recipients by 25%.
A major clinical trial has found the change in intravenous fluid therapy with balanced crystalloid solution could deliver real benefits for kidney transplant recipients.
The BEST-Fluids trial was conducted by the Australasian Kidney Trials Network (AKTN) in collaboration with researchers from the University of Queensland (UQ), the University of Adelaide, and the University of Sydney.
The trial assessed the use of an intravenous fluid containing sodium, potassium, magnesium and chloride in proportions similar to human blood instead of the usual practice of using normal saline.
“Of those study participants who received intravenous Plasma-Lyte 148, 30% needed dialysis after their transplants, compared to 40% for those given normal saline,” ATKN chair and UQ Professor David Johnson said.
“This is a significant improvement in outcomes for those undergoing kidney transplant surgery.”
Patients who receive a kidney transplant from a deceased donor are at risk of delayed graft function (DGF), which is defined as the requirement for dialysis within the first week after transplantation due to poor kidney function.
The research findings found those who received balanced crystalloid solution rather than saline were at lower risk of DGF – with approximately one case of DGF prevented for every 10 patients treated.
The available evidence suggests that balanced crystalloid solution should be the standard of care intravenous fluid used in deceased donor kidney transplantation, say the AKTN researchers.
Study lead Dr Michael Collins, Nephrologist at the Royal Adelaide Hospital, said the findings could change world practice for kidney treatments.
“Based on these results, we believe that balanced crystalloid fluids should be used for all patients during and after kidney transplant surgery,” he said.
“Balanced fluids are relatively cheap and widely available, which makes them a useful treatment for most of the estimated 200,000 kidney transplant operations that are conducted globally each year.”
In current practice using regular saline, about one in three transplanted kidneys do not work immediately, said University of Sydney Professor and Senior Investigator Steve Chadban.
“Kidneys can be effectively ‘stunned’ by the traumas of donation surgery, transportation and then transplant surgery,” he said.
“When a kidney doesn’t work immediately, dialysis is required. This can cause anxiety and inconvenience for patients, as well as potential longer-term damage to the kidney.”
More than 800 transplant recipients participated in the study, across 16 hospitals in Australia and New Zealand between January 2018 and August 2020.
The double-blinded trial was funded from the Medical Research Future Fund in Australia and the Health Research Council of New Zealand, and supported by the South Australian Health and Medical Research Institute.
The research is published in The Lancet.