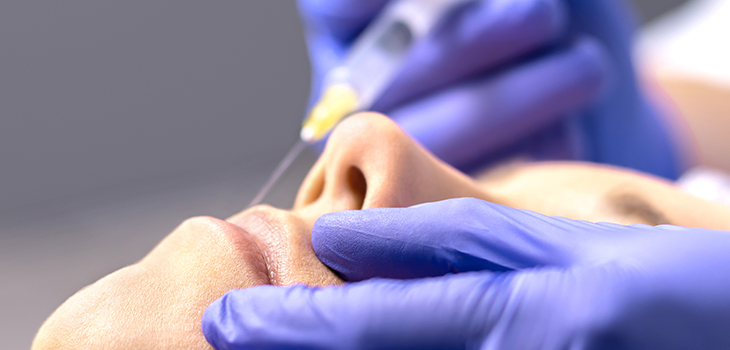
Registered nurses will now need at least one-year of general or specialist practice before they can perform non-surgical cosmetic procedures, under new guidelines taking effect from today.
Many practitioners who want to expand their scope of practice into non-surgical cosmetic procedures, which include botulinum toxin and dermal fillers, will also need to undertake further training or education.
The Australian Health Practitioner Regulation Agency (Ahpra) and National Boards published the Guidelines for practitioners who perform non-surgical cosmetic procedures and the Guidelines for practitioners who advertise higher risk non-surgical cosmetic procedures as part of sweeping changes aimed at strengthening safeguards across the industry.
On top of new expectations for all registered health practitioners, young people will be better protected, with those under 18 considering non-surgical cosmetic procedures to undergo a mandatory seven-day cooling off period between their first consultation and any procedures. Advertising by practitioners aimed at those under 18 will be banned.
The Guidelines include specific advice for nurses and midwives about the Nursing and Midwifery Board of Australia’s (NMBA’s) requirements and expectations about the education and experience of nurses and midwives considering providing non-surgical cosmetic procedures.
For example, enrolled nurses, who must work under the supervision of an RN, wanting to practise in the area of non-surgical cosmetic procedures will be required to have practised for a minimum of one-year post initial registration, have one-year experience in a related area of practice (dermatology, general surgery) prior to practising in non-surgical cosmetic procedures, and completed education and training relevant to practise in the area.
According to the Guidelines, ENs working in the field must:
- Not undertake the administration of dermal filler injectables to very high-risk areas, including the glabella, nose and forehead.
- only undertake the administration of dermal filler injectables to high-risk areas, including temples, nasolabial folds, peri-orbital and medial cheek, in a clinical setting where there is immediate onsite access to the prescriber of the cosmetic injectable and/or an RN, and
- only perform laser skin resurfacing with direct supervision of an RN who checks the laser settings before use.
NMBA Chair Adjunct Professor Veronica Casey described the new guidelines as the robust response needed to protect consumers accessing the growing industry.
“A lot of people might think of these procedures in the same way they’d consider getting a facial or a haircut. But these are clinical procedures and require appropriate training and experience to be performed safely,” Adjunct Professor Casey said.
Read the new Guidelines here