The Australian Government has detailed a national rollout strategy for the delivery of COVID-19 vaccines. In Phase 1a, vaccines will be available to quarantine and border workers, priority sub-groups of frontline health workers, and aged care and disability care staff and residents.1
Any COVID-19 vaccine approved for use in Australia may be effective in reducing the severity of illness but may not completely protect against infection or prevent a person from transmitting the virus to others. All current official recommendations regarding infection prevention and control should continue to be observed regardless of vaccine status.
*ALERT* Evidence regarding COVID-19 is continually evolving. This resource will be updated regularly to reflect new emerging evidence but may not always include the very latest evidence in real-time.
To read or download a .PDF of the information contained in this article, please follow this link: ANMF COVID-19 Resources
Key points
- Any vaccine used in Australia must be approved by the Therapeutic Goods Administration (TGA).
- The Pfizer/BioNtech mRNA vaccine and the Oxford/AstraZeneca viral vector vaccines have provisional TGA approval in Australia.
- The Novavax protein subunit vaccine is under consideration for approval in Australia.
- These COVID-19 vaccines have been found to be effective for reducing the severity of COVID-19 infections (Pfizer/BioNtech 90%, Oxford/AstraZeneca 70%, Novavax 95.6%).
- The vaccines do not ‘cure’ COVID-19 or completely prevent infection.
- Research continues to investigate how effective the vaccines are for reducing the severity of illness caused by SARS-CoV-2 variants (e.g. United Kingdom and South African variants).
- The vaccines cannot cause COVID-19 infection or change human DNA.
- Vaccination symptoms are local or systemic reactions to foreign particles, not a ‘mild’ form of COVID-19.
- The evidence is unclear regarding whether COVID-19 vaccines reduce transmission.
- Vaccines train the immune system to more quickly recognise and effectively respond to COVID-19 infection.
- The Pfizer/BioNtech vaccine contains viral mRNA for specific spike proteins not complete or viable viruses.
- A person who is vaccinated may still be infected by COVID-19 before or after vaccination and may still transmit the virus to others.
- Vaccinated people must still follow official guidance and recommendations regarding infection prevention and control (i.e. hand hygiene, respiratory etiquette, physical distance).
Agreements, registration, and approval
Many vaccines for COVID-19 are currently under development at varying stages.2 Australia currently has agreements in place with four COVID-19 vaccine developers (Table 1).3
Table 1: Australian Government COVID-19 Vaccine agreements
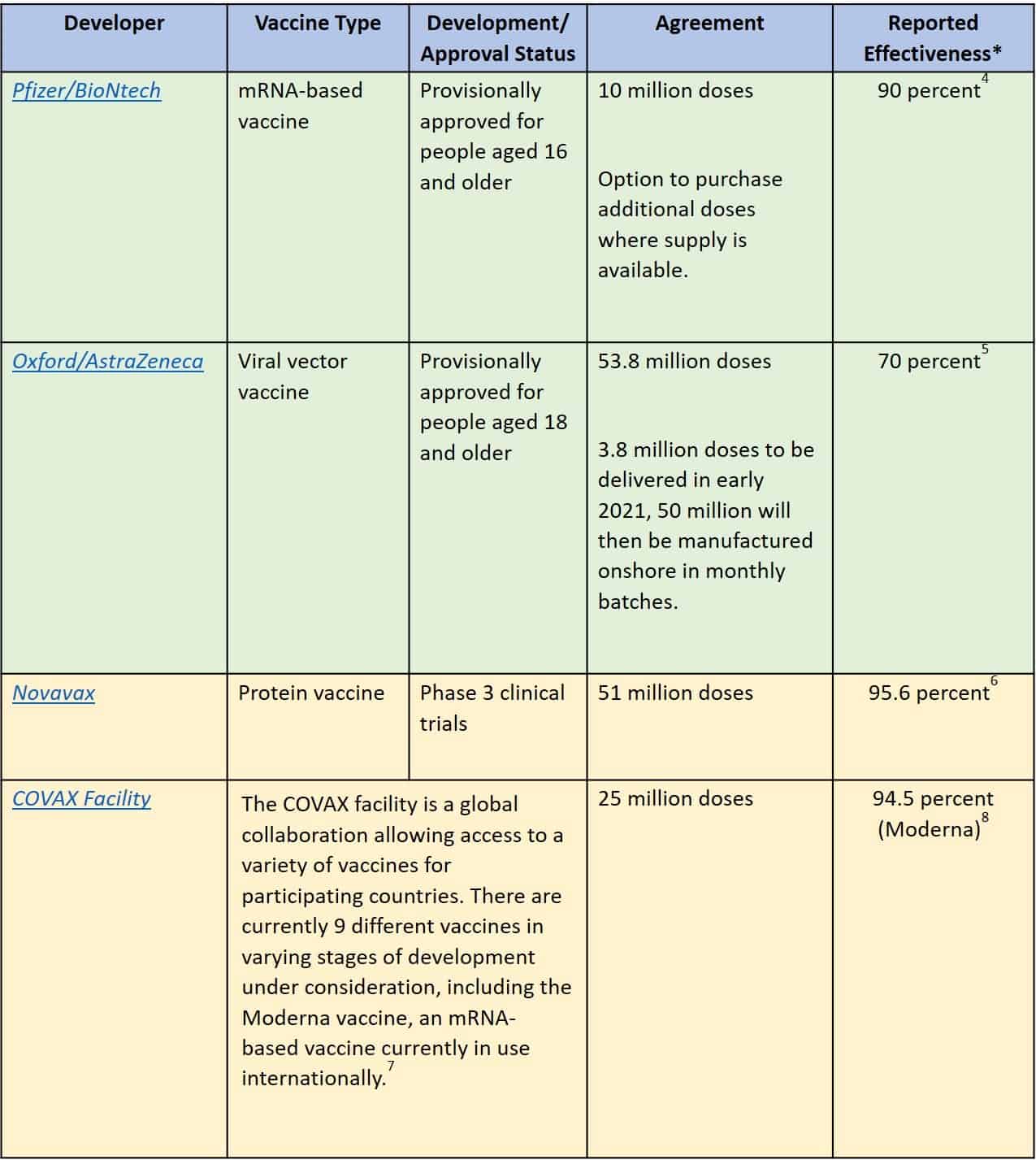
*Reports of vaccine effectiveness vary across publications and may change rapidly as new evidence emerges.
Only vaccines that are approved as safe and effective by the Australian Therapeutic Goods Administration (TGA) and granted provisional registration will be available in Australia. Safety and effectiveness is determined through analysis of ongoing clinical trials, international collaboration, and advice from the Advisory Committee on Vaccines (ACV). The TGA will continue to monitor the safety, quality, and efficacy of all vaccines before and following provisional approval.9,10
How COVID-19 vaccines work
Each COVID-19 vaccine falls into one of several categories of how the vaccine is developed and causes an immune response.11
Viral infection and the immune response
COVID-19 is caused by the SARS-CoV-2 virus. Viruses, including SARS-CoV-2, cannot self-replicate and need a host cell to reproduce and cause infection. SARS-CoV-2 viruses are coated in ‘spike proteins’ that bind to ‘ACE2 receptor proteins’ which are located on the surface of many cells within the human body (see Figure 1). When the spike protein and receptor proteins bind, the virus can enter the cell and replicate resulting in infection.12,13
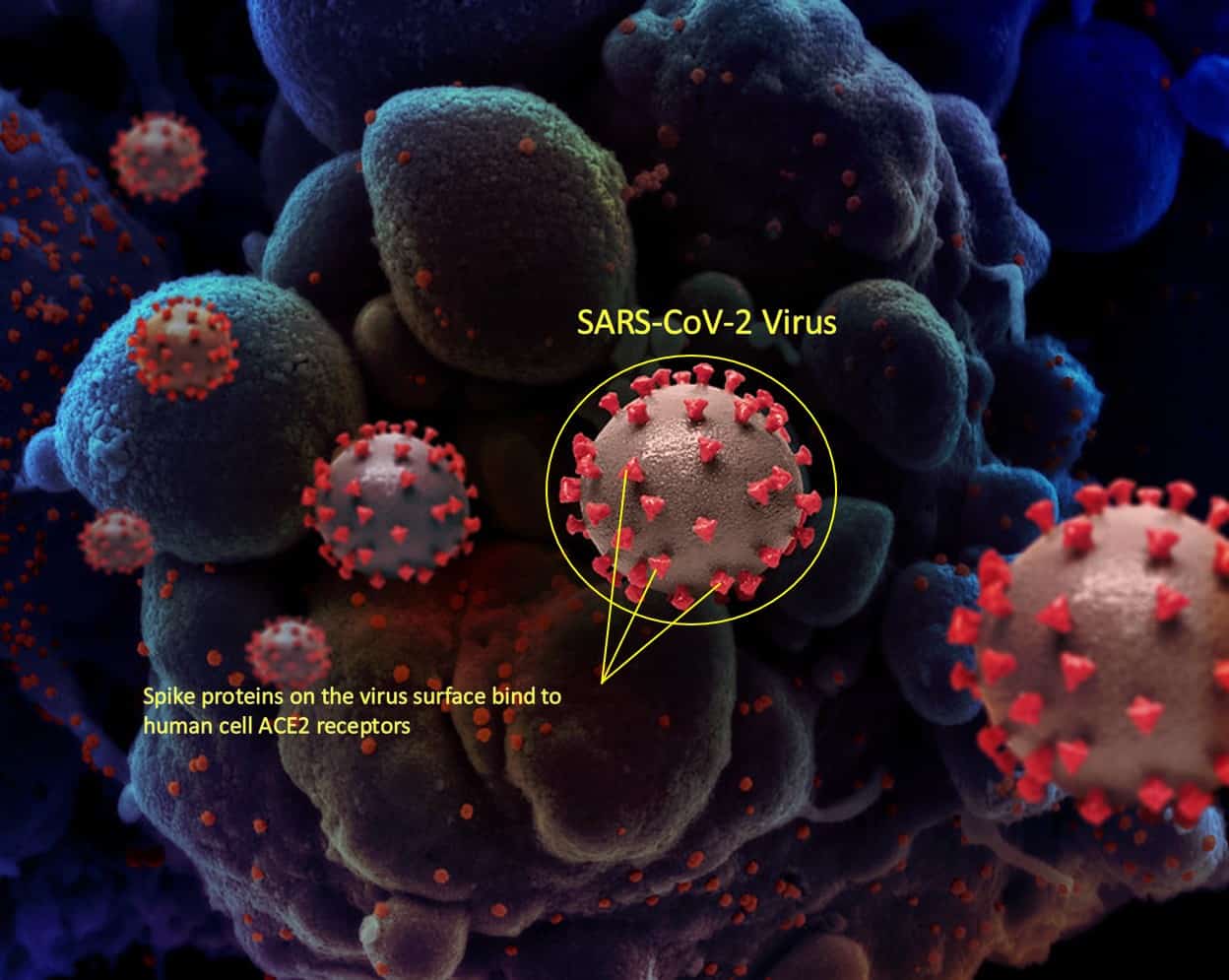
Figure 1: Unscaled depiction of the SARS-CoV-2 virus. The virus cells (circled) use the spike protein to attach to the surface of the body’s cells (blue, in background). The spike protein is shaped somewhat like a crown (corona) and gives the virus its name.[*]
To reduce or prevent infection, the body’s immune system must recognise the virus. To do this ‘B-cells’ produce antibodies that bind to SARS-CoV-2 spike proteins (see Figure 2). This then prevents the virus from binding to other human cells. Simultaneously, ‘T-cells’ identify and destroy infected cells and prevent further infection. Destroyed cells are consumed by macrophages and purged from the body.14
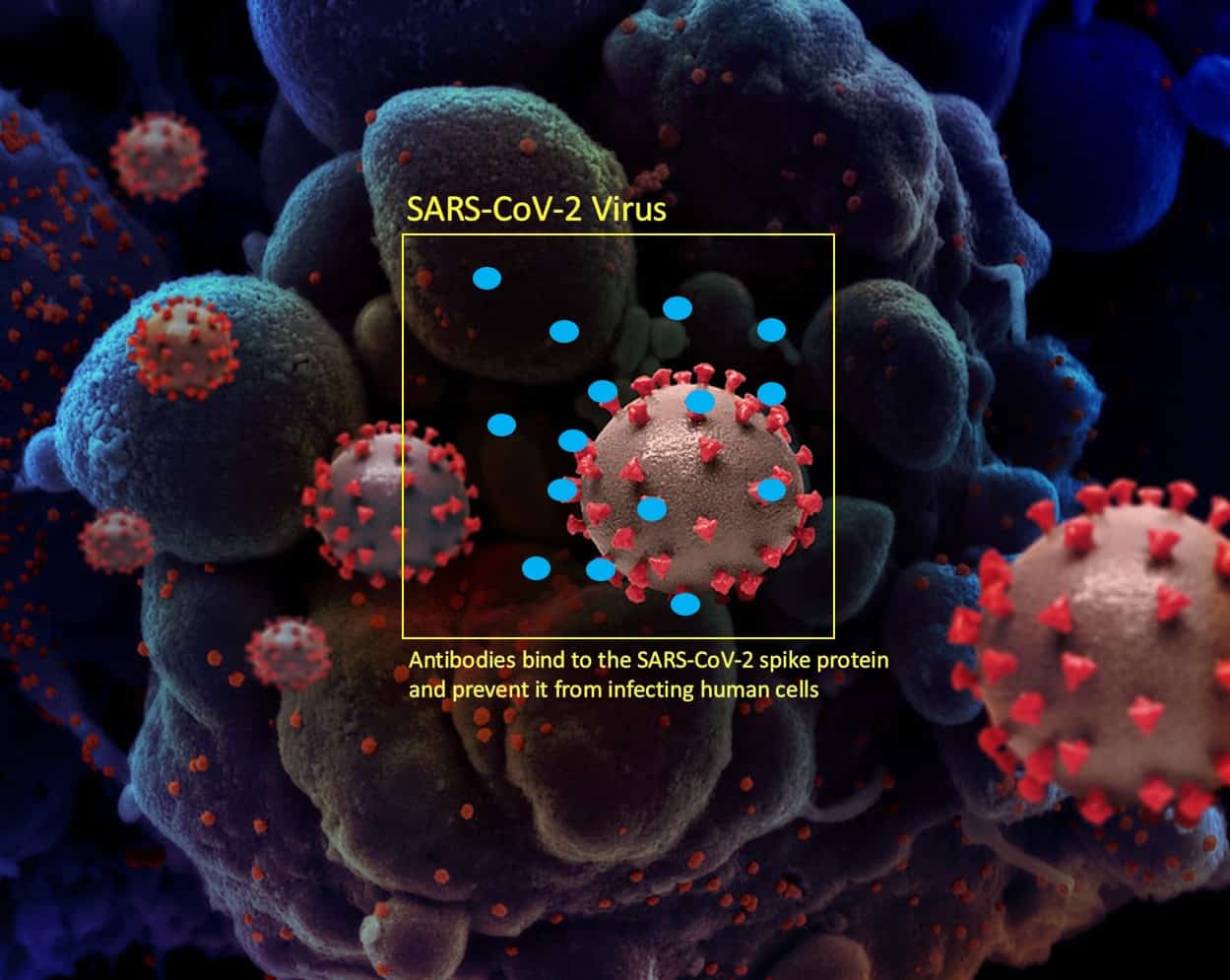
Figure 2: Unscaled depiction of the SARS-CoV-2 virus with human antibodies (blue) produced by the bodies B-Cells attaching to the virus’ spike protein preventing the virus from attaching to and infecting human cells.[*]
[*] Image adapted from images published by the National Institute of Allergy and Infectious Diseases (NIAID). Novel Coronavirus SARS-CoV-2. NIAID. Available from: https://www.flickr.com/photos/niaid/50022374313/in/album-72157712914621487/
The effectiveness of a person’s immune response to SARS-CoV-2 depends on the ability of their body to quickly recognise and respond to viral spike proteins and infected cells. Once the immune system is able to do this, the B- and T-cells remain within the body and continue to provide protection against future infection for some time.14
While some vaccines for other illnesses use a weakened or harmless version of the virus,[‡]15 COVID-19 vaccines isolate and use the SARS-CoV-2 spike protein to generate an immune response.16 When the body recognises the spike protein in the vaccine, B- and T-cells react, programming themselves for recognition of the full virus. These cells are then present to inhibit future infection and reproduction of the SARS-CoV-2 virus if or when it is encountered by the body.14
[‡] A live attenuated vaccine is a complete but genetically weakened version of a virus. ‘Inactivated’ vaccines are similar in that they contain whole viruses, however these are rendered ‘dead’ prior to administration.
SARS-CoV-2 reproduction
The SARS-CoV-2 virus includes 29 proteins which have been identified with high-confidence as responsible for 332 protein-human interactions across multiple biological processes. Each protein is required for the virus to function properly and infect human host cells.17
During normal viral reproduction each of these proteins is translated from the virus’s genetic code (ribonucleic acid – RNA). Messenger ribonucleic acid (or mRNA) are subsets of genetic code that carry instructions for the reproduction of individual proteins.12
The COVID-19 vaccines incorporate either only the limited sequence of RNA or mRNA that codes for a single harmless spike protein – not all of the virus’s genetic information. This means that the vaccines cannot cause COVID-19 infection or make someone infectious.
mRNA Vaccines (eg. Pfizer/BioNtech and Moderna vaccines)
Vaccines using mRNA vaccine technology have been in development for other viruses (e.g. Zika), but before now have not been used in practice.15,18
The Pfizer/BioNtech vaccine and Moderna vaccine are mRNA vaccines given in two doses (Pfizer/BioNtech 21 days apart,19 Moderna 28 days apart).20 The vaccines contain the mRNA of a harmless SARS-CoV-2 spike protein responsible for binding the virus to human cells.21 When administered, the body’s cells translate the mRNA and produce spike proteins. These spike proteins are identified by the body’s immune system which then begins developing antibodies to the SARS-CoV-2 virus.16
The Pfizer/BioNtech vaccine is the only mRNA vaccine currently approved by the TGA in Australia. Because the mRNA in the Pfizer/BioNtech vaccine degrades easily, it requires very cold transport and storage temperatures (-70°C±10°C for up to 10 days unopened).19 The vaccine can be stored for up to five days at 2-8°C, but cannot be refrozen.19 The Moderna vaccine is transported frozen at between -25°C and -15°C,22 and appears to be able to be stored at 2-8°C for up to 30 days and may be kept for up to eight hours at room temperature in a syringe.20
Viral vector vaccine (eg. Oxford/AstraZeneca vaccine)
The Oxford/AstraZeneca viral vector vaccine is administered in two doses with a 4 to 12 weeks interval (12 weeks is recommended where possible).23,24 Viral vector vaccines use harmless viral non-SARS-CoV-2 cells to introduce the genetic sequence that codes for the SARS-CoV-2 spike protein into the body (as opposed to only the mRNA). In the case of the Oxford/AstraZeneca vaccine this is a harmless chimpanzee adenovirus.5,25
Once introduced, the body’s cells begin production of the harmless SARS-CoV-2 spike protein without causing COVID-19 or viral vector replication.26 The presence of this spike protein trains an immune response to the SARS-CoV-2 virus. Other viral vector vaccines include those for Ebola, Hepatitis B, Human Papilloma Virus, and Whooping Cough.25
The Oxford/AstraZeneca vaccine can tolerate temperatures of 2-8°C for at least six months.24,27
Protein vaccine (eg. Novavax vaccine)
If approved by the TGA the Novavax protein vaccine (SARS-CoV-2 recombinant spike protein vaccine) will likely be administered in two doses 21 days apart and may be stored, transported, and handled at 2-8°C.28 These vaccines are developed by engineering a harmless insect baculovirus to contain genetic information for the SARS-CoV-2 spike protein.29 Cultured insect cells are infected with the baculovirus which then produce SARS-CoV-2 spike proteins.30 The spike protein units are isolated and combined with an adjuvant which enhances antibody production.31,32 When administered, an immune response to the SARS-CoV-2 virus is triggered.
Genetic mutations
As commonly occurs with other viruses (e.g. influenza), new variants of the SARS-CoV-2 virus have arisen through natural genetic mutation.33 Variants that have altered spike proteins unlike those of the original virus may be less susceptible to current vaccines.34 This is because the antibodies produced by the body based on the original SARS-CoV-2 variant may not bind as effectively to differently shaped spike proteins on the surface of new variants.35 Research continues into establishing how well existing vaccines work to develop an effective immune response and reduce the severity of illness caused by variants.33 The vaccine development process can be readily modified to accommodate new virus strains in the same manner as for influenza.36,37
Reactions to vaccines
Because COVID-19 vaccines do not introduce fully functional viruses, reactions to the vaccine are not a “mild form” of COVID-19. Reactions (reactogenicity) are the immune system’s response to the introduction of a foreign body (i.e. the isolated SARS-CoV-2 spike proteins or viral vector cells). It is not possible for the vaccine to infect a person with COVID-19 or cause changes to human DNA.22 Severe anaphylactic reactions to the Pfizer/BioNtech Oxford/AstraZeneca vaccines appear to be uncommon.38,39
Reactogenicity can include local injection site pain, redness, swelling, and other more systemic symptoms such as fever, muscle soreness, fatigue, or headache.40 For both the Pfizer/BioNtech and AstraZeneca vaccines approved in Australia, most reactions are mild (i.e. do not interfere with daily activities) and only last a day or two. Moderate to severe reactions (i.e. headache, fever/chills, fatigue) are very uncommon and usually also resolve in two to three days.25,41
Vaccines and transmission
Although evidence shows that existing COVID-19 vaccines can reduce the severity of COVID-19 infection, the impact of the vaccines on infectiousness/transmission of SARS-CoV-2 between people is still unclear.42 The collection of data regarding the vaccines, particularly in the face of variant strains, is ongoing, and in some cases yet to be released.21,25,32
Because it has not been specifically studied extensively, it is largely unknown how the vaccines may impact transmissibility of the virus.42 Further, because the vaccines do not offer 100 percent protection from the virus, a person may still be infected just prior to or after vaccination and still pass on the infection to others even when asymptomatic or with only mild symptoms.43
While infection from contaminated surfaces appears to be rare,44 vaccination also does not prevent individuals from physically transferring the virus from contaminated surfaces. It is therefore important that even vaccinated people adhere to current official recommendations regarding infection prevention and control to prevent the possible spread of SARS-CoV-2.45
References
- Australian Government Department of Health. Australia’s COVID-19 vaccine national roll-out strategy 2021. https://www.health.gov.au/resources/publications/australias-covid-19-vaccine-national-roll-out-strategy (accessed Feb 8 2021).
- Forni G, Mantovani A, Forni G, et al. COVID-19 vaccines: where we stand and challenges ahead. Cell Death & Differentiation 2021; 28(2): 626-39.
- Australian Government Department of Health. Australia’s vaccine agreements 2021. https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/about-covid-19-vaccines/australias-vaccine-agreements (accessed Feb 8 2021).
- Pfizer. Pfizer and BioNTech Announce Vaccine Candidate Against COVID-19 Achieved Success in First Interim Analysis from Phase 3 Study. Feb 9 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against (accessed Feb 16 2021).
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet 2021; 397(10269): 99-111.
- Mahase E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ 2021; 372: n296.
- European Centres for Disease Prevention and Control. Overview of the implementation of COVID-19 vaccination strategies and vaccine deployment plans in the EU/EEA. 2021. https://www.ecdc.europa.eu/sites/default/files/documents/Overview-of-COVID-19-vaccination-strategies-deployment-plans-in-the-EU-EEA.pdf (accessed Feb 8 2021).
- Moderna. Moderna’s COVID-19 Vaccine Candidate Meets its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. Feb 16 2021. https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy (accessed Feb 16 2021).
- Therapeutic Goods Administration (TGA). COVID-19 vaccines. 2021. https://www.tga.gov.au/covid-19-vaccines (accessed 8 Feb 2021).
- Australian Government Department of Health. Australian COVID-19 Vaccination Policy 2020. https://www.health.gov.au/resources/publications/australian-covid-19-vaccination-policy (accessed 8 Feb 2021).
- World Health Organization (WHO). The different types of COVID-19 vaccines. Jan 12 2021. https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained (accessed Feb 16 2020).
- V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nature Reviews Microbiology 2020.
- Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367(6483): 1260-3.
- Sewell HF, Agius RM, Kendrick D, Stewart M. Covid-19 vaccines: delivering protective immunity. BMJ 2020; 371: m4838.
- Krammer F. SARS-CoV-2 vaccines in development. Nature 2020; 586(7830): 516-27.
- Walsh EE, Frenck RW, Falsey AR, et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. New England Journal of Medicine 2020; 383(25): 2439-50.
- Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020; 583(7816): 459-68.
- Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics — developing a new class of drugs. Nature Reviews Drug Discovery 2014; 13(10): 759-80.
- Pfizer. Pfizer-BioNTech COVID-19 Vaccine U.S. Distribution Fact Sheet. 20 November 2020 2020. https://www.pfizer.com/news/hot-topics/covid_19_vaccine_u_s_distribution_fact_sheet (accessed Feb 16 2021).
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine 2020; 384(5): 403-16.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 2020; 383(27): 2603-15.
- United States Centers for Disease Control and Prevention (CDC). Moderna COVID-19 Vaccine. Dec 22 2020. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html (accessed Feb 16 2021).
- Wise J. Covid-19: New data on Oxford AstraZeneca vaccine backs 12 week dosing interval. BMJ 2021; 372: n326.
- AstraZeneca. COVID-19 Vaccine AstraZeneca confirms 100% protection against severe disease, hospitalisation and death in the primary analysis of Phase III trials. 3 Feb 2021. https://www.astrazeneca.com/media-centre/press-releases/2021/covid-19-vaccine-astrazeneca-confirms-protection-against-severe-disease-hospitalisation-and-death-in-the-primary-analysis-of-phase-iii-trials.html#! (accessed Feb 16 2021).
- Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet 2020; 396(10267): 1979-93.
- United States Centers for Disease Control and Prevention (CDC). Understanding and Explaining Viral Vector COVID-19 Vaccines. Jan 5 2021. https://www.cdc.gov/vaccines/covid-19/hcp/viral-vector-vaccine-basics.html#:~:text=Viral%20vector%20vaccines%20use%20a,is%20used%20as%20the%20vector. (accessed Feb 16 2021).
- Knoll MD, Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. The Lancet 2021; 397(10269): 72-4.
- Say D, Crawford N. Novavax COVID-19 vaccine. Jan 5 2021. https://mvec.mcri.edu.au/references/novavax-covid-19-vaccine/ (accessed Feb 16 2021).
- Yang J, Wang W, Chen Z, et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020; 586(7830): 572-7.
- Wang J. New strategy for COVID-19 vaccination: targeting the receptor-binding domain of the SARS-CoV-2 spike protein. Cellular & Molecular Immunology 2021; 18(2): 243-4.
- Reimer JM, Karlsson KH, Lövgren-Bengtsson K, Magnusson SE, Fuentes A, Stertman L. Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS One 2012; 7(7): e41451.
- Keech C, Albert G, Cho I, et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. New England Journal of Medicine 2020; 383(24): 2320-32.
- Koyama T, Weeraratne D, Snowdon JL, Parida L. Emergence of Drift Variants That May Affect COVID-19 Vaccine Development and Antibody Treatment. Pathogens 2020; 9(5).
- Fiorentini S, Messali S, Zani A, et al. First detection of SARS-CoV-2 spike protein N501 mutation in Italy in August, 2020. The Lancet Infectious Diseases.
- Conti P, Caraffa A, Gallenga CE, et al. The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem. J Biol Regul Homeost Agents 2020; 35(1).
- Bollinger R, Ray S. New Variants of Coronavirus: What You Should Know. Jan 29 2021. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/a-new-strain-of-coronavirus-what-you-should-know (accessed Feb 16 2021).
- Kim JH, Marks F, Clemens JD. Looking beyond COVID-19 vaccine phase 3 trials. Nature Medicine 2021; 27(2): 205-11.
- Shimabukuro T, Nair N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021.
- Glover RE, Urquhart R, Lukawska J, Blumenthal KG. Vaccinating against covid-19 in people who report allergies. BMJ 2021; 372: n120.
- Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019; 4(1): 39.
- United States Centers for Disease Control and Prevention (CDC). What to Expect after Getting a COVID-19 Vaccine. Feb 12 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/expect/after.html (accessed Feb 16 2021).
- Peiris M, Leung GM. What can we expect from first-generation COVID-19 vaccines? The Lancet 2020; 396(10261): 1467-9.
- Johansson MA, Quandelacy TM, Kada S, et al. SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Network Open 2021; 4(1): e2035057-e.
- Goldman E. Exaggerated risk of transmission of COVID-19 by fomites. The Lancet Infectious Diseases 2020; 20(8): 892-3.
- Iboi EA, Ngonghala CN, Gumel AB. Will an imperfect vaccine curtail the COVID-19 pandemic in the U.S.? Infectious Disease Modelling 2020; 5: 510-24.