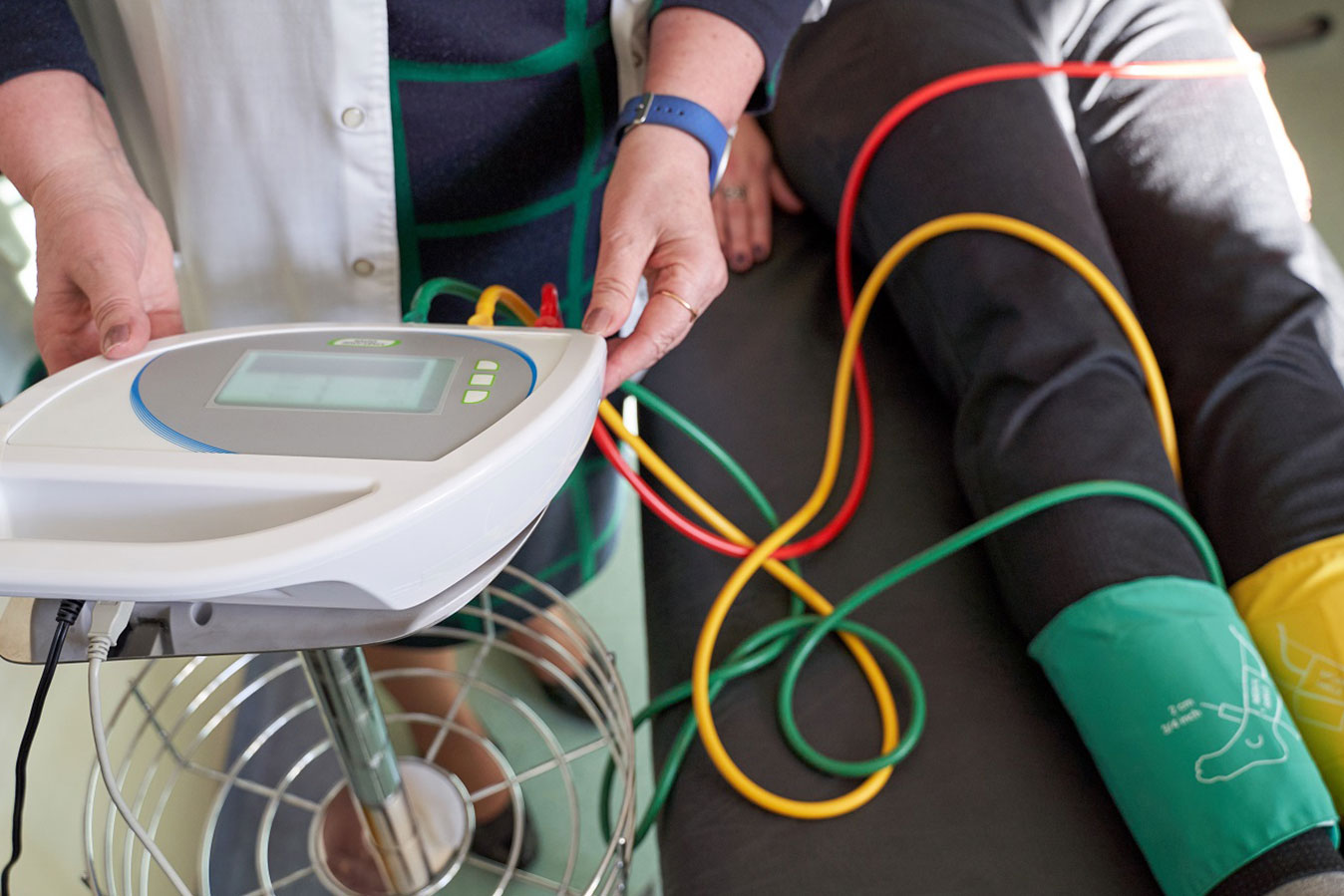
Patients with peripheral artery disease (PAD) with blocked lower leg arteries could soon have access to treatment with a dissolvable stent which has been shown to be more effective than standard balloon angioplasty.
A phase 3 randomised clinical trial (RCT) led by UNSW researchers found that in patients treated with the dissolvable stent Esprit BTK, their occluded blood vessel was more likely to remain open, reducing the need for limb amputation.
Principal Investigator Conjoint Associate Professor Ramon Varcoe, from UNSW Medicine & Health and the Prince of Wales Hospital, said Esprit BTK would expand treatment options for patients suffering from PAD.
“The prognosis after amputation is worse than most cancers,” said Associate Professor Varcoe, a clinical academic and vascular surgeon.
The researchers are working with the Therapeutic Goods Administration (TGA) Authorised Prescriber Scheme in an attempt to gain early access for patients at the Prince of Wales Hospital before the device is approved for widespread use in Australia.
According to the Australian Institute for Health and Welfare, PAD was the primary cause of almost 60,000 hospitalisations in 2020-21. PAD most commonly affects the lower limbs.
The build-up of plaque in severe PAD leads to chronic limb-threatening ischaemia (CTLI). Patients with CTLI experience severe pain, gangrene, non-healing ulcers and may require limb amputation, with poor survival rates.
When PAD occurs in the blood vessels below the knee, the current standard of care is balloon angioplasty.
“Currently, we use angioplasty but this has no mechanical scaffolding properties and usually doesn’t stay open very long,” said Associate Professor Varcoe.
“In many cases, after balloon angioplasty, the treated blood vessel becomes narrower and the balloon doesn’t stay inflated for long enough. This results in the blood vessel becoming blocked again over time.”
There is potential to use stents for below-the-knee PAD. However, no such devices have been approved yet for this purpose, with several failed clinical trials.
Indicated for the treatment of blocked arteries below-the-knee, Esprit BTK is a mechanical scaffold made from poly-l-lactic acid, which safely dissolves in the body over 18-24 months.
“The Esprit scaffold gives mechanical support to the recently opened artery, it delivers a drug to the blood vessel wall that prevents re-narrowing, and then it dissolves, leaving nothing behind to irritate the blood vessel and induce scar tissue formation,” said Associate Professor Varcoe.
“It also avoids burning bridges should the patient require bypass surgery in the future.”
The Phase 3 RCT for Esprit BTK involved 261 patients globally with blocked lower leg arteries, who were at risk of requiring limb amputation. These patients were treated with either balloon angioplasty or Esprit BTK.
At the one-year timepoint after treatment, the researchers found that Esprit BTK was much more effective at keeping the artery open, critical for preventing the need for limb amputation.
About 75% of the Esprit BTK group experienced this outcome, compared to 44% of the balloon angioplasty group.
There was little difference between the two treatments in safety profile. Serious adverse events occurred in 3% of patients in the balloon angioplasty group and 2% in the Esprit BTK group.
“This is the first positive RCT in this space and it gives us an effective tool to save limbs in this group of patients who are often very sick,” said Associate Professor Varcoe.
“Once approved, I expect it will become the standard of care.”
Following the success of the Phase 3 RCT, Esprit BTK will be submitted to the Food and Drug Administration (FDA) for approval in the US initially, followed by further submissions in Europe, Japan and Australia.
The clinical trial findings were published in the New England Journal of Medicine.