Changing the way commonly used antibiotics are given to adult patients with sepsis will save thousands of lives a year globally, according to research by the University of Queensland and The George Institute for Global Health
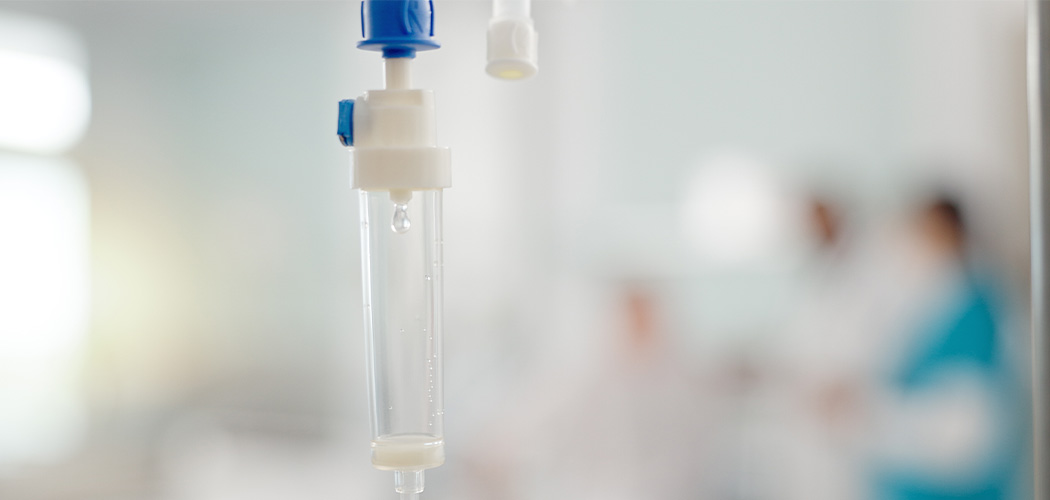
A clinical trial and systematic review have shown that intravenously administering commonly used β-lactam antibiotics via continuous infusion – instead of multiple short infusions – cures infections and saves lives.
The clinical trial of more than 7,000 patients tested findings from laboratory studies to deliver the best drug concentration for the bacteria causing the infection.
Prolonged infusion was defined as either an extended infusion (intravenous β-lactam antibiotic administration for 2 hours or longer during a dosing interval) or a continuous infusion (constant intravenous β-lactam antibiotic administration that could be administered as a sequential 6-, 8-, 12-, or 24-hour infusion).
Intermittent infusion was defined as intravenous β-lactam antibiotic administration for fewer than 2 hours during a dosing interval.
“We found by delivering these antibiotic doses as a continuous (prolonged) infusion we can maintain the concentration of the antibiotic in a patient’s blood and tissue, and kill bacteria at a greater rate,” said Professor Jason Roberts, Director of UQ’s Centre for Clinical Research (UQCCR) and Metro North Health’s Herston Infectious Diseases Institute.
“This simple intervention uses commonly available antibiotics, so even small hospitals in third-world countries can implement the dosing change almost as easily as well-resourced hospitals in developed countries,” he said.
The international trial BLING (Beta-Lactam Infusion Group) III was one of the largest ever antibiotic randomised clinical trials. The trial, sponsored by The George Institute, involved 104 hospitals in seven countries and more than 130,000 doses of medication.
The clinical trial data was then used in a systematic review and meta-analysis, combining 18 studies and more than 9000 patients.
“The combined data showed a very significant benefit with the use of a continuous infusion, saving one life for every 26 patients treated,” said Associate Professor Naomi Hammond, Critical Care Program Head at The George Institute.
The next step for the research team would be to inform worldwide treatment protocols and guidelines.
“Physicians follow international guidelines when treating sepsis patients, and at the moment these guidelines have a very low certainty of evidence around how to best administer these drugs,” said UQCCR Emeritus Professor Jeffrey Lipman.
“Thanks to our program of research, treatment protocols and guidelines will now have a very high certainty.
“Given the simple nature of the findings and the conversations we are having between hospitals, we expect most will adopt these changes immediately.”
The research is the culmination of a program of work over 20 years led by Emeritus Professor Lipman that began with small studies focusing on dosing, to large clinical trials with multinational collaborators.
The BLING III trial was funded by the National Health and Medical Research Council of Australia, the Belgian Healthcare Knowledge Centre (KCE), the Health Research Council of New Zealand, The University of Queensland, University Hospital Nimes, Skane University Hospital, UK National Institute for Health and Care Research.
The Royal Brisbane and Women’s Hospital Foundation provided funding for the program of research, which commenced with the BLING I trial. The BLING III trial involved collaborators from Australia, New Zealand, Malaysia, Sweden, Belgium, France and the UK.
The research was published in two papers, here and here, in The Journal of the American Medical Association (JAMA).